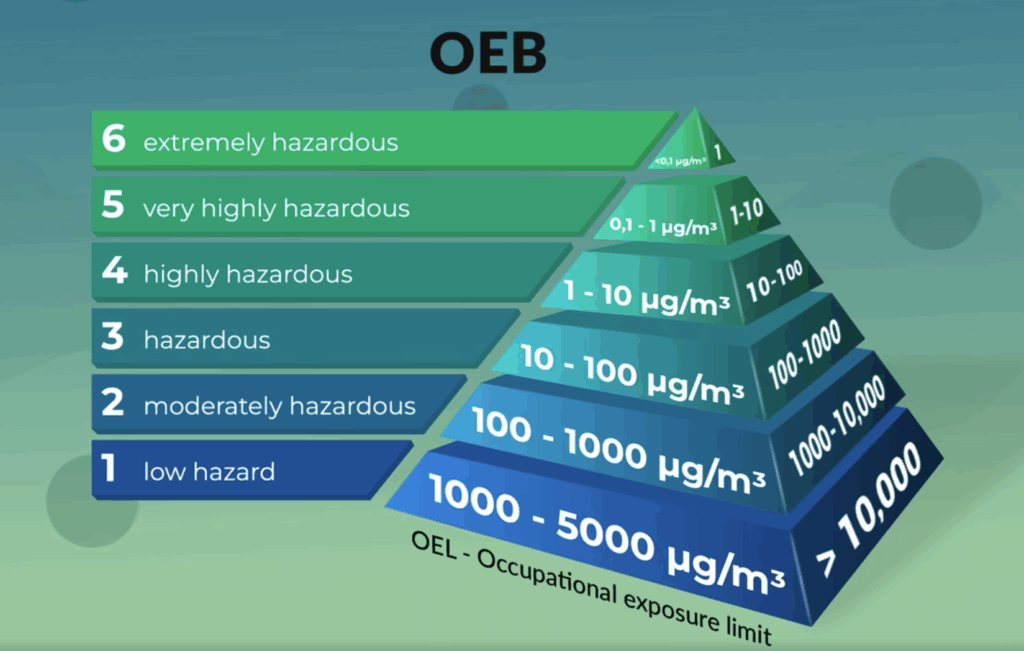
At Sever Pharma Solutions, we have the experience, expertise, and facilities required to work with your high-potent substances in a safe manner. This includes manufacturing pharmaceutical products containing APIs that are ranked in the highest occupational exposure bands (OEB) up to and including OEB6 . For this purpose, we have policies, procedures, and capabilities in place, allowing us to handle high-potent drugs in small and large quantities to meet your needs in the challenging high-potent drug development and manufacturing field.
Our focus on safety starts immediately in early development, and we maintain this focus throughout the entire development. For you, this means that you can rely on Sever Pharma Solutions to develop safe and sustainable processes for manufacturing dosage forms containing highly potent APIs.
Within Sever Pharma Solutions, we manufacture products containing high-potent APIs daily in our labs and manufacturing facilities. For this reason, the development of safe manufacturing processes and a safe working environment is paramount, and the following aspects are part of our comprehensive approach:
To ensure that all three above-mentioned safety and environmental aspects are met, a coherent Safety Health and Environment (SHE) strategy has been implemented at Sever Pharma Solutions, which is based on the following elements:
New projects related to high-potent drugs start with initiating a safety, health, and environmental (SHE) risk assessment, using customer and generally available data to identify the most appropriate way(s) to mitigate the risks. The activities resulting from this risk assessment become integral to the project plan, which will be generated in close collaboration with the customer.